FFRIntas stands as a pharmaceutical powerhouse that has revolutionized the global healthcare landscape through innovative formulation, manufacturing, and marketing strategies. This comprehensive platform serves as a gateway for healthcare professionals, researchers, and industry stakeholders to access cutting-edge pharmaceutical resources and tools that drive medical advancement worldwide.
Understanding FFRIntas: A Pharmaceutical Leader
FFRIntas has established itself as a dominant force in the pharmaceutical industry, operating under the prestigious Accord Healthcare brand to serve diverse international markets. The company’s foundation rests on addressing critical healthcare challenges through a comprehensive drug value chain that encompasses every aspect of pharmaceutical development and distribution.
The organization’s commitment to excellence is evident in its vertically integrated business model, which maintains strict quality control throughout the entire production process. This comprehensive approach allows FFRIntas to deliver consistent, high-quality pharmaceutical products that meet stringent international standards while addressing unmet clinical and societal requirements.
Step-by-Step FFRIntas Login Process for 2025
Accessing your FFRIntas account has been streamlined to ensure a seamless user experience for healthcare professionals and authorized personnel. The login process remains consistent and user-friendly for 2025:
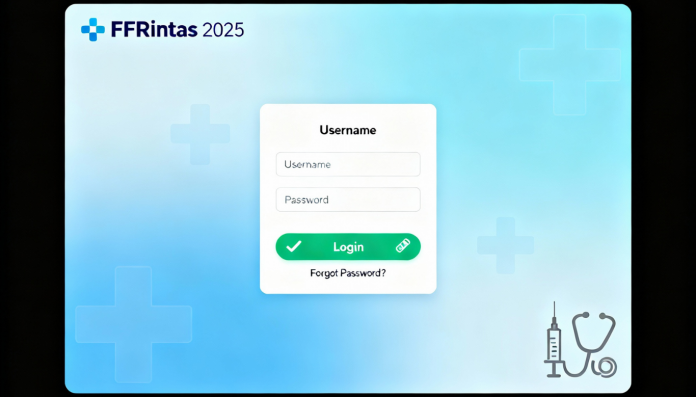
Website Navigation: Begin by visiting the official FFRIntas portal at newffr.intaspharma.com/IntasFFR, which serves as the dedicated login gateway for all authorized users. This secure platform ensures protected access to valuable pharmaceutical resources and proprietary information.
Credential Entry: The login interface features clearly marked fields for your username and password. Accuracy is crucial when entering these credentials to prevent authentication issues and ensure smooth access to your account. The system implements robust security measures to protect sensitive pharmaceutical data and user information.
Account Authentication: After entering your credentials, click the login icon positioned on the right side of the interface. The system will verify your information against its secure database, granting access to personalized dashboards, research tools, and pharmaceutical resources tailored to your professional requirements.
This streamlined authentication process reflects FFRIntas’s commitment to user convenience while maintaining the highest security standards required in the pharmaceutical industry.
Historical Foundation and Growth Trajectory
FFRIntas has built its reputation through strategic vision and consistent execution since its establishment. The company’s foundation was laid with a clear mission to address healthcare challenges on a global scale, operating under the Accord Healthcare brand to effectively serve international markets.
The organization’s growth story exemplifies organic development combined with strategic acquisitions, significantly expanding its product portfolio and market reach. This dual approach has enabled FFRIntas to establish a strong presence across North America, Europe, and Asia, positioning itself as a truly global pharmaceutical leader.
Investment in research and development has been a cornerstone of FFRIntas’s success, leading to breakthrough innovations that have strengthened its competitive position. The company has consistently allocated substantial resources to R&D activities, resulting in a robust pipeline of pharmaceutical products that address critical healthcare needs.
Regulatory Excellence and Quality Assurance
FFRIntas’s commitment to quality is demonstrated through rigorous adherence to international regulatory standards. The company has successfully obtained approvals from prestigious regulatory agencies, including the FDA, EMA, and TGA, ensuring that all products meet the highest quality benchmarks.
This regulatory compliance extends beyond mere approval acquisition to encompass comprehensive quality management systems that govern every aspect of pharmaceutical development and manufacturing. The company’s facilities undergo regular inspections and audits to maintain certification standards and ensure continuous compliance with evolving regulatory requirements.
The organization’s quality assurance framework incorporates advanced monitoring systems, standardized operating procedures, and continuous improvement protocols that guarantee product integrity from conception to market delivery.
Comprehensive Business Operations
The Intas Corporation operates as a vertically integrated pharmaceutical entity, encompassing all aspects of drug development, manufacturing, and distribution. This comprehensive business model ensures seamless coordination across different operational phases while maintaining quality control throughout the entire value chain.
State-of-the-art manufacturing facilities equipped with cutting-edge technology enable FFRIntas to produce diverse pharmaceutical products, including generics, biosimilars, and specialty medicines. These facilities incorporate advanced automation systems, quality control laboratories, and environmental management protocols that ensure optimal production conditions.
The company’s operational structure supports efficient resource allocation, risk management, and strategic decision-making across multiple therapeutic areas and geographic markets. This integrated approach facilitates rapid response to market demands while maintaining consistent product quality and regulatory compliance.
Innovation and Future-Focused Strategies
FFRIntas is actively pursuing innovative solutions that will shape the future of pharmaceutical care and healthcare delivery. The company’s strategic focus encompasses several key areas that promise significant impact on global health outcomes.
Biosimilar Development: FFRIntas is making substantial investments in biosimilar development, recognizing the growing demand for cost-effective biologic alternatives. These biosimilars offer highly similar therapeutic profiles to approved reference products while providing more accessible pricing options for healthcare systems worldwide.
New Chemical Entities (NCEs): The organization is committed to developing novel chemical compounds that can provide enhanced therapeutic outcomes for patients across various disease categories. This focus on NCE development represents FFRIntas’s dedication to advancing pharmaceutical science and addressing unmet medical needs.
Technological Integration: The company leverages advanced technological solutions to optimize manufacturing processes, incorporating automation and sophisticated data analytics to enhance efficiency and reduce production costs. These technological advancements enable more precise quality control and faster time-to-market for new pharmaceutical products.
Sustainability Initiatives: FFRIntas demonstrates corporate responsibility through comprehensive sustainability programs that explore eco-friendly manufacturing practices and supply chain management. These initiatives aim to minimize environmental impact while maintaining operational efficiency and product quality.
Employee Development and Organizational Culture
FFRIntas recognizes that employee empowerment is fundamental to achieving organizational excellence and maximizing potential across all business sectors. The company’s human resource strategy emphasizes creating a culture of informed decision-making and professional growth.
Training and development programs are designed to attract and retain top industry talent while fostering continuous learning and skill enhancement. These programs ensure that employees remain current with evolving pharmaceutical technologies, regulatory requirements, and industry best practices.
The organization’s collaborative culture encourages innovation, cross-functional teamwork, and knowledge sharing, creating an environment where employees can contribute meaningfully to the company’s mission of improving global healthcare outcomes.
Market Position and Global Reach
FFRIntas has established strong market positions across multiple geographic regions through strategic planning and consistent execution. The company’s global network, branded as Accord Healthcare, demonstrates its commitment to quality pharmaceutical services and customer satisfaction.
Market expansion strategies focus on identifying underserved therapeutic areas and geographic regions where FFRIntas can make meaningful contributions to healthcare improvement. This approach ensures sustainable growth while addressing genuine medical needs in diverse populations.
The company’s competitive advantages include its vertically integrated operations, robust regulatory compliance, innovative product pipeline, and commitment to quality excellence, positioning it favorably against industry competitors.
Conclusion: Leading Pharmaceutical Innovation
FFRIntas represents the evolution of modern pharmaceutical excellence, combining traditional quality principles with innovative approaches to healthcare delivery. The user-friendly FFRIntas login system provides seamless access to comprehensive pharmaceutical resources and tools that support healthcare professionals worldwide.
As the company continues navigating future challenges and opportunities, its unwavering commitment to quality, innovation, and employee empowerment will drive continued success in the global pharmaceutical market. FFRIntas’s dedication to addressing unmet clinical needs and improving healthcare outcomes positions it as a leader in shaping the future of pharmaceutical care and medical advancement.